Businesses within the pharmaceutical and life sciences sector must continuously ensure batch quality is maintained at the highest standards.
After all, quality is the most critical metric in pharmaceutical manufacturing, nothing is more important than protecting patient health. However, the impact doesn’t go without also reaching bottom-lines and profitability. The numbers speak for themselves: The cost of a single batch deviation can range from $20,000 to $1M per batch, depending on the product.
Tight control of processes, inputs, and other variables is a necessity for successful pharmaceutical manufacturing. Traditionally, there have not been effective ways of looking at historical and time-series data to investigate deviations and variability besides spending painfully tedious hours of subject matter expert (SME) time in spreadsheets. Engineers look to create process parameter profiles to serve as guides for reducing process variability and increasing yield for all future batch development—also known as the “golden batch profile.”
But there are two problems with this. First, creating golden batch profiles repeatedly requires many hours spent manually sifting through years of data or delayed lab results that make it difficult to optimize process inputs to control the batch yield. And second, out-of-tolerance events will still occur, regardless of applying diligence in controlling the Critical Process Parameters (CPPs) of a recipe, as measured by a group of Critical Quality Attributes (CQAs). Often, it becomes clear the number of variables and the cause-and-effect relationships connecting these two aspects are more complex than originally assumed.
Find Your “Golden Batch Profile”—Efficiently
The data is there. But it’s time to efficiently analyze it. The method of manually extracting production data from historians and various repositories within an industrial control system and creating graphs in Excel is outdated and doesn’t solve the whole puzzle of accurately finding the relationships mentioned above. There are many limitations on how a spreadsheet can actually be applied to understand complex process variability and provide actionable insights. Leading pharmaceutical companies have made the transition to advanced analytics to find their perfect batch parameters.
Applying Advanced Analytics to Make Data-Backed Decisions
The most efficient and intuitive way to lead your team to golden batch discovery and application is through advanced analytics. Applying the technology eliminates manual work in spreadsheets and enables easy data cleansing, contextualization, aggregation, and analysis of process data in near real-time. By making a live connection to all applicable data sources, SMEs can spend less time gathering data and manually aligning time stamps -- freeing up their time to apply the analysis to the process parameters and production methods to see improvements in quality and performance.
An advanced analytics platform for process manufacturing, can be applied across your entire organization, running in a browser with live connections to process historians to quickly extract data and present results.
A Behind-the-Scenes Look
To visualize the application in action and for this specific business issue, assume you’re examining a production process with six CPPs connected to a single unit procedure. Using historical data from ideal batches with acceptable specifications on all CQAs, advanced analytics enables you to simply and easily graph these six variables from all the previous unit procedures. Curves representing performance from historical CPPs can then be superimposed on top of each other using identical scales to reveal new insights within the application.
It’s immediately seen if the curves tend to form a tight group, or if they are spread out, showing different values at various times. Use of advanced analytics platform can easily aggregate these curves without the need for complex formulas or macros to establish an ideal profile for each CPP. Engineers can replicate this procedure, resulting in an updated reference profile and boundary for every variable. In the end, this process reveals new opportunities for process optimization.
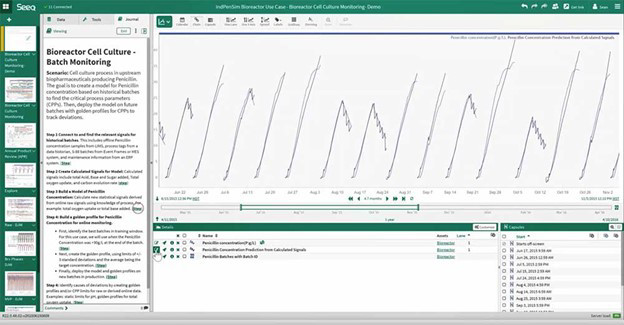
In the screenshot below, advanced analytics is analyzing the cell culture process in an upstream biopharmaceutical manufacturer that is producing Penicillin. The technology is used to create a model for Penicillin concentration based on historical batches to find the CPPs that will produce the ideal batch. This model can then be deployed on future batches with golden batch profiles for CPPs to effectively track deviations and prevent them from occurring.